Implementation Research:
Translating the ABCS into HIV Care
Get Ready and Empowered About Treatment
(GREAT 2)
Use of highly effective, often single pill HIV antiretroviral therapy has dramatically reduced deaths from AIDS-related causes yielding an aging population among people living with HIV (PLH) who increasingly experience cardiovascular (CVD) morbidity and mortality. Age and sex-adjusted deaths from CVD are appreciably higher among PLH than among the general population. This growing CVD risk among PLH can be substantively reduced through use of established interventions, commonly referred to as ABCS, i.e. appropriate use of aspirin, blood pressure control, cholesterol reduction, and smoking cessation, in addition to lifestyle, i.e. diet, physical activity, and safe drinking. Each of these interventions is underused among PLH. There is a fundamental gap in scientific knowledge regarding which strategies will promote shared decision-making regarding ABCS between PLH and their clinicians. Addressing this gap is vital to reducing the growing CVD burden among PLH.
The overall goal of this project is to develop and rigorously test implementation strategies to address this gap in scientific knowledge. We will test the following patient-, clinician-, and practice-level strategies: patient/activation training and texting support, clinician-targeted academic detailing and audit and feedback, and practice-based engagement in a stepped wedge randomized controlled trial (RCT) in 600 PLH across 9 practices in Rochester, NY, New York City and Dallas, Texas. We hypothesize that use of evidence-based, multilevel implementation strategies will improve discussion and uptake of the ABCS among PLH.
Intervention Components
Each enrolled clinician will participate in the designated intervention activities over the course of eight months (which is the standard intervention duration for each wedge):
Two Academic Detailing (AD) Sessions
Four Online CME Modules
Two Audit Feedback Reports
Academic Detailing (AD) Sessions
Aspirin for ASCVD Prevention
Individualizing risks and shared decision making are essential when considering aspirin for ASCVD prevention. While the literature suggests people living with HIV may be at increased risk of ASCVD, evidence regarding aspirin for primary or secondary prevention in this population is limited. Therefore data from the general population must be used to guide best practice recommendations.
Key Message 1
Aspirin is recommended for secondary prevention of ASCVD.
Key Message 2
Evidence for aspirin supporting primary ASCVD prevention is comparatively weak. Individual 10-year risk of ASCVD should be carefully considered alongside potential for aspirin-related bleeds before recommending aspirin for primary prevention.
Key Message 3
The online risk calculator (and app) is available to estimate an individualized risks and benefits of aspirin for primary prevention.
Aspirin for ASCVD Prevention Management Algorithm
- Older age
- Male gender
- Smoker
- Diabetes
- History of bleeding
- Hypertension
- Peptic ulcer disease
- Use of NSAIDs or anticoagulants
Blood Pressure Management
HIV and antiretroviral medications increase potential for hypertension and CV events. Optimal blood pressure control while minimizing potential for drug interactions is an essential component of HIV care.
Key Message 1
Stage I hypertension is defined as a systolic blood pressure of 130 – 139 mmHg or a diastolic blood pressure of 80 – 89 mmHg. For patients with stage I hypertension and a 10-year ASCVD risk, 3 – 6 months of lifestyle management should be attempted prior to starting pharmacotherapy. For those with a 10-year risk ≥10%, CVD, diabetes, or renal dysfunction, one antihypertensive agent should be initiated.
Key Message 2
Stage II hypertension is defined as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg. Two antihypertensive agents from different classes should be initiated in patients with stage II hypertension along with lifestyle changes.
Key Message 3
The pooled cohort calculator may underestimate ASCVD risk among PLH; true risk may be 50 – 100% higher than that estimated by the calculator. Therefore pharmacologic treatment may be appropriate for PLH with stage I HTN and a 10-year risk <10%.
Key Message 4
ACE-Inhibitors, ARBs, dihydropyridine calcium channel blockers, and thiazide/thiazide like diuretics are considered first line therapies. Blood pressure should be assessed monthly after initiation and the regimen should be adjusted as needed to achieve a goal blood pressure of <130/80 mmHg.
|
||||
|
||||
|
||||
|
Expect 1 mmHg reduction for every 1 kg lost!
- Aerobic exercise (150 min/week): -5 to -8 mmHg
- Resistance training (150 mg/week): -4 mmHg
- Isometric hand grips (4 x 2 min, 3 times weekly for 8 – 10 weeks): -5 mmHg
- Alcohol intake (≤2 drinks/day for men and ≤1 drink/day for women): -4mmHg
Pharmacotherapy for Hypertension
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Use of Antihypertensive & Antiretrovirals
| Beta Blockers | Bictegravir Dolutegravir Raltegravir | No adjustments necessary. | |
| Elvitegravir/ Cobicistat | Beta blocker dose may need to be decreased. Potential for interaction may be minimized by using atenolol, labetalol, nadolol, or sotolal. | ||
| Calcium Channel Blockers | Bictegravir | No adjustments necessary. | |
| Dolutegravir Raltegravir | No adjustments necessary. | ||
| Elvitegravir/ Cobicistat | Co-administer with caution. Slowly titrate calcium channel blocker dose and monitor for side effects and toxicities. | ||
| Dihydropyridine Calcium Channel Blockers | Efavirenz Etravirine Nevirapine | Titrate calcium channel blocker based on clinical response. | |
| Diltiazem, Verapamil | Efavirenz | Titrate diltiazem of verapamil based on clinical response. | |
| Beta Blockers | All protease inhibitors | Beta blocker dose may need to be decreased. Potential for interaction may be minimized by using atenolol, labetalol, nadolol, or sotolal. | |
Cholesterol Management
Cholesterol management is an important part of reducing CVD risk among persons living with HIV. The ACC/AHA Cholesterol Guidelines were updated in 2018 to allow for more individualized treatment interventions, detailed risk assessment (including the introduction of risk-enhancing factors) and thresholds for starting non-statin agents (see Cholesterol Algorithm below) in those at the highest risk for ASCVD as follows.
Key Message 1
Patients with existing ASCVD, history of multiple major ASCVD events, or 1 major ASCVD event, and multiple high-risk conditions, should be prescribed statin therapy unless contraindicated for secondary prevention.
Key Message 2
Patients 40 to 75 years of age with severe hypercholesterolemia (LDL-C ≥190 mg/dL) or who have diabetes and an LDL-C ≥ 70 mg/dL, should be started on statin therapy without further need for calculating 10-year ASCVD risk.
Key Message 3
Patients 40 to 75 years of age without clear indication for statin therapy (i.e., clinical ASCVD, very-high risk for ASCVD, severe hypercholesterolemia, or elevated LDL-C and diabetes) should be evaluated for primary ASCVD prevention using a risk scoring tool to help guide shared decision-making for management.
Drug-drug interactions between statins and antiretrovirals must be considered during prescribing.
Key Message 4
PLH who are/will be prescribed ARVs should have a careful evaluation for drug-drug interactions prior to the initiation of statin (or ARV) therapy.
ARV-Statin Drug-Drug Interactions
| Statins | ||||||||||||||||||||
| Atorvastatin | ||||||||||||||||||||
| Fluvastatin | ||||||||||||||||||||
| Lovastatin | ||||||||||||||||||||
| Pitavastatin | ||||||||||||||||||||
| Pravastatin | ||||||||||||||||||||
| Rosuvastatin | ||||||||||||||||||||
| Simvastatin |
| KEY | No clinically significant interaction expected | ↑ | Potential increased exposure of the statin | |
| These drugs should not be coadministered | ↓ | Potential decreased exposure of the statin | ||
| Potential interaction which may require dose adjustment or close monitoring | ↔ | No significant effect | ||
| Potential interaction of weak intensity. No a priori dose adjustment recommended |
Cholesterol Management Algorithm
Treatment
Goals of Therapy
- Age ≥65 years
- Heart failure
- Smoker
- Diabetes
- Prior CABG or PCI
- Hypertension
- Chronic kidney disease
- LDL persistently ≥100 mg/dL
- Heterozygous familial hyperlipidemia
- Family history of early ASCVD
- LDL persistently ≥160 mg/dL or TG ≥175 mg/dL
- Chronic kidney disease
- Metabolic syndrome
- Inflammatory conditions (RA, psoriasis, HIV) Ethnicity (South Asian) Conditions in women that increase CV risk (e.g, menopause <40)
Smoking Cessation for ASCVD Prevention
One in three patients with HIV smokes and smoking accounts for about 60% of deaths among people living with HIV. Though the benefits of smoking cessation are clear, the literature suggests evidence-based treatment for smoking is underutilized, particularly among HIV patients.
Key Message 1
The 5 A’s is a quick and effective method to assess smoking cessation readiness and provide counseling.
Key Message 2
The 4 R’s is a useful method to prompt contemplation among smokers who are not yet ready to quit.
Key Message 3
Pharmacotherapy increases quit success rate. Combination nicotine replacement therapy, varenicline, and bupropion are considered first line and selection should be guided by patient preference and comorbidities.
The 5 A’s for Smoking Cessation
- Ask about current and prior tobacco use.
- Prior smokers: Congratulate and counsel on triggers avoidance to prevent relapse.
- Current smokers: Assess frequency of use, types of tobacco product(s) used, and prior quit attempts.
- Advise current users to quit and provide clear, strong, and personalized suggestions to help ensure success.
- Assess willingness to quit.
- If patient is not ready, move to the 5 R’s.
- Assist patients ready to quit in setting a quit date in ☆ near future (2 – 4 weeks). Encourage them to share date with friends/family and request support.
- Offer pharmacotherapy.
- Schedule follow up 1 – 2 weeks after quit date. ☆
- Remember relapses are common and the majority of those who relapse are ready to make another quit attempt within 30 days.
The 5 R’s for Patients Not Ready to Quit
- Collaborate with patient to identify why quitting is personally relevant to them (i.e. comorbidities, cost, other life goals).
- Ask patient to explain their perceived risks of smoking.
- Ask patient to identify benefits of quitting smoking.
- Determine patient’s barriers to quitting. Remind patient that many tobacco users make repeat quit attempts before they are successful.
- Repeat process at each visit
- In 20 min: HR and BP drop
- In 12 hrs: CO level return to normal
- In 2 weeks – 3 months: circulation & lung function improve
- In 1 year: risk of CHD is reduced by half
- In 5 years: stroke risk is the same as non-smokers
|
|
|
|
|
|
||
|
|
|
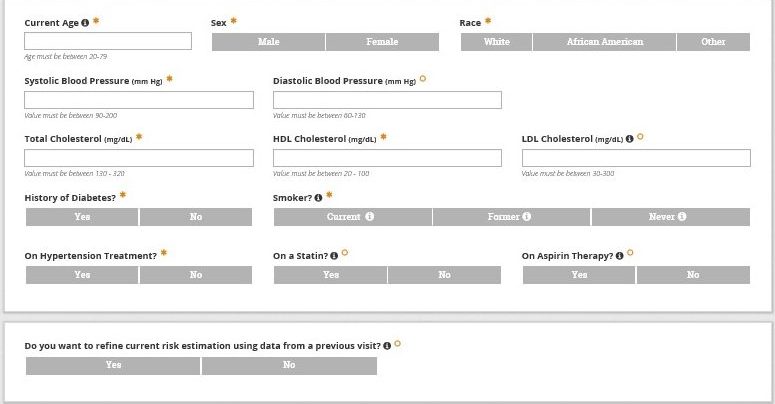
Assessing ASCVD Risk
Use of an ASCVD risk calculator improves risk assessment beyond clinician estimates. In the absence of formal risk calculation, clinician’s estimates are inaccurate and tend to underestimate ASCVD risk. In practice, clinicians tend to emphasize one or two risk factors and fail to appreciate the impact of compounded risk. To avoid underutilization of primary prevention strategies consider the following.
Key Message 1
Assessing ASCVD risk among PLH is best done using handheld or online risk calculation using the ACC/AHA ASCVD Risk Estimator Plus.
Key Message 2
Clinicians should recognize that HIV infection enhances risk for ASCVD meaning that actual risk often exceeds that calculated based on traditional risk factors.
Key Message 3
Use the ACC/AHA ASCVD Risk Estimator Plus for shared decision making with patients regarding ASCVD risk reduction using the ABCS (Aspirin, Blood pressure control, Cholesterol management, and Smoking cessation) based on National Guidelines for each.
ASCVD Primary Prevention Pearls
Adults age 40-75 years being evaluated for CV disease prevention should undergo 10-year ASCVD risk estimation and have a clinician-patient risk discussion before starting pharmacologic therapy.
Use of risk calculation improves risk assessment beyond clinician estimates and results in greater reduction in risk factors without clinical harm. Assess ASCVD risk at an initial visit with a patient to establish a reference point and forecast the impact of various interventions on patient risk and reassess after implementing ABCs.
Assess for other risk enhancing factors to help guide decisions about primary prevention interventions in select individuals. HIV infection is a “risk-enhancing” factor associated with increased risk of an ASCVD event, impacting the 10-year risk estimate. HIV often increases risk 50-100% beyond calculated risk.
| Risk-Enhancing Factors |
|
|---|
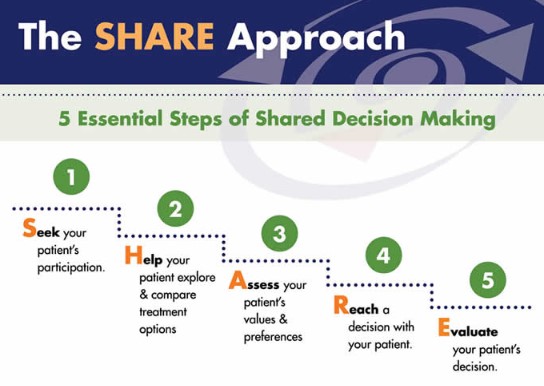
Shared Decision-Making
Resources and Tools
Inclusion Criteria
Patient- Age 40-75 years by 7/1/2018
- HIV/AIDS diagnosis
- Receipt of care within the participating practice
- At ≥5% risk for CVD as calculated using the ASCVD Risk Estimator Plus
- Current lipid values, i.e. within 15 months of enrollment
- Own a texting capable cellphone
- Proficient in English or Spanish and is willing to participate
- Expects to remain in the study throughout the entire study period
- Physician, nurse practitioner, or physician assistant who provides direct HIV care within a participating practice and is willing to participate
- Serve a cohort of at least 100 HIV patients, have an Electronic Health Record (EHR) and agree to collaborate on implementing feasible adaptations of these strategies
Exclusion Criteria
Patient- Current participation in other CVD trials
- Plans to leave the practice within 12 months
- Pregnant
- Experienced a prior cardiac or vascular event such as myocardial infarction (MI) or cerebrovascular accident (CVA) or have had a CVD procedure such as installation of a stent or angioplasty
- Have peripheral vascular disease
- Intermittent claudication or peripheral arterial disease
- Lack capacity to consent
- Plans to leave the practice within 12 months
- Aspirin Therapy
- Blood Pressure
- Cholesterol
- Smoking Cessation
- Medication Adherence
- Assessing ASCVD
COVID-19 & Commonly Prescribed Drugs
HIV A B C S NEWSLETTER